Qlosi Experience
Delivering moments designed for comfort
Developed with the lowest effective concentration of pilocarpine to minimize side effects experienced by presbyopia patients1

Well-established safety and tolerability profile across 5 clinical trials1,2:
-
No serious adverse events occurred with Qlosi
-
The most commonly reported adverse events were instillation site pain and headache (5%-8%)
| |
Instillation site pain may resolve in seconds |
 |
In patients who reported mild headache, symptoms resolved in less than an hour, and in some patients resolution was significantly faster |
-
Qlosi is contraindicated in presbyopia patients with known hypersensitivity to the active ingredient or to any of the excipients
- Qlosi is not contraindicated for use with presbyopia patients who have had refractive surgical procedures, like LASIK, or cataract surgery
Qlosi gave patients a comfortable experience1
Safety that stands apart
Across clinical trials, every Qlosi adverse event stayed in the single digits
Qlosi demonstrated single-digit incidence for all reported adverse events across clinical studies.
Qlosi1,2
(0.3% Pilocarpine)
Vuity"3,6"
(1.06% Pilocarpine)
VIZZTM7,8*
(1.44% Aceclidine)
Headache
4.8% moderate
16% moderate or severe
4% severe, 7% moderate
Instillation site pain/irritation
Blurred vision
Visual impairment
Conjunctival hyperemia
Ocular hyperemia
*Most common adverse reactions defined as adverse reactions reported in ≥5% of patients.
†Dim vision reported.
AEs=adverse events
VIZZ™ is a trademark of LENZ Therapeutics, Inc. VUITY® is a registered trademark of AbbVie Inc.
Qlosi’s unique 0.4% concentration was specifically designed to optimize near-vision efficacy and minimize side effects.1,2,9,10
See more
Qlosi’s safety profile has remained consistent across clinical studies and real-world use, with 0 reported cases of retinal detachment (RD) to date.1
Risk of retinal detachment (RD) is not unique to Qlosi—it’s a rare class effect associated with all miotics.1,11 Patients should be evaluated prior to prescribing.1,3,4,11
Qlosi’s lower concentration and controlled dosing help minimize the risk factors historically seen with older, higher-dose therapies.1,3,7
RD concerns are typically linked to factors not associated with Qlosi12:
-
Historical use of higher-concentration miotics
-
Initial use by non-ECPs using inconsistent patient monitoring
In clinical trials of Qlosi1:
-
No cases of RD were reported
In early real-world use13:
- No RD cases have been reported in the first 11 months of availability13
Examination of the retina is advised prior to initiating therapy with any miotic.
New data demonstrates Qlosi is pupil selective14
Similar ciliary muscle movement compared to balanced salt solution (BSS) control
Data from a prospective, head-to-head clinical study conducted at the Bascom Palmer Eye Institute. Thousands of high-resolution images of the anterior segment were obtained to assess the impact of low vs high concentrations of pilocarpine (Qlosi 0.4% vs 2%), at various focal lengths, as compared to BSS control.
Pilocarpine behaves in a concentration-dependent manner on the ciliary muscle during accommodation
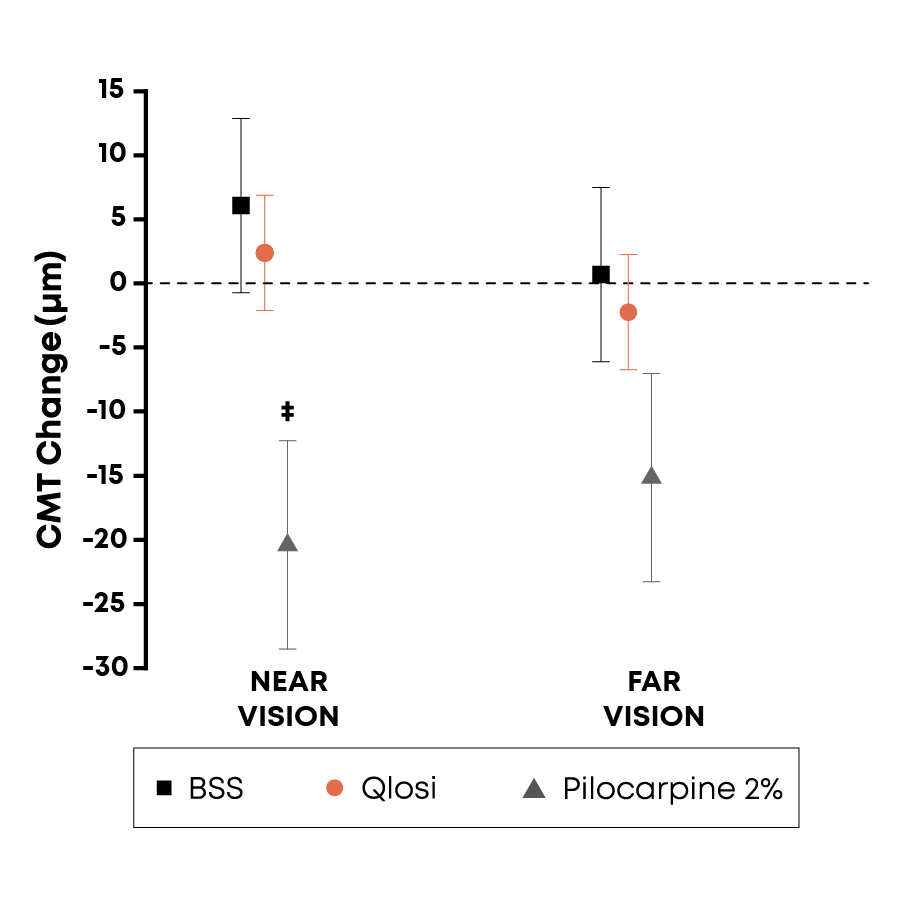
‡p<0.05; Error bars=±1 SE.
Source: Bascom Palmer Eye Institute. January 2026
Study design: This was a prospective, fixed-sequence crossover design with masked data analysis. The study enrolled 10 patients and processed >10K images.
Qlosi, low-concentration 0.4% pilocarpine: comparable to BSS with no statistically significant change in ciliary muscle thickness (CMT)
High-concentration (2%) pilocarpine: demonstrated significant changes in CMT during accommodation
SE=standard error
Qlosi, 0.4% pilocarpine. The concentration formulation designed for presbyopia.14
Qlosi is pupil selective, supporting accommodative demands with minimal ciliary muscle engagement.
This can potentially reduce tractional forces associated with a high‑concentration (2% pilocarpine) miotic.
“So this data shows that Qlosi is ciliary [muscle] sparing, and that significantly reduces, in my opinion, the risk of retinal detachment. It gives me lot of confidence to have a conversation that really sets accurate expectations.”
- Dr. Harmin Chima, OD | Cleveland OH