Efficacy
First-line clear near vision with Qlosi, day or night*
Provide your presbyopia patients more clear near moments without compromising distance vision1,2
Nearly all patients experienced enhanced functional near vision (20/40 or better) on Day 153

Clear near vision—without compromising distance vision2
Clinical data from Day 8 and Day 15 demonstrate a consistent combination of efficacy, safety, and comfort, resulting in meaningful improvements in near vision1,2
40% of presbyopia patients on Qlosi achieved a ≥3-line improvement in DCNVA, and no loss of 1-line or more in distance visual acuity on Day 8 at 1 hour post-dose 1 vs those taking Vehicle (19%) (p<0.0001)2
DCNVA=distance-corrected near visual acuity
Day or night.*
8 out of 10
patients taking Qlosi experienced enhanced functional near vision (20/40 or better) on Day 15 with binocular vision3
*Advise patients to exercise caution in night driving and other hazardous activities in poor illumination.
After 2 doses of Qlosi, functional near vision was extended for up to 8 hours3
Day 15—Subjects with DCNVA 20/40 or better in Study Eye (Monocular) vs Binocular Vision with all baseline subjects with 20/40 or better OU removed
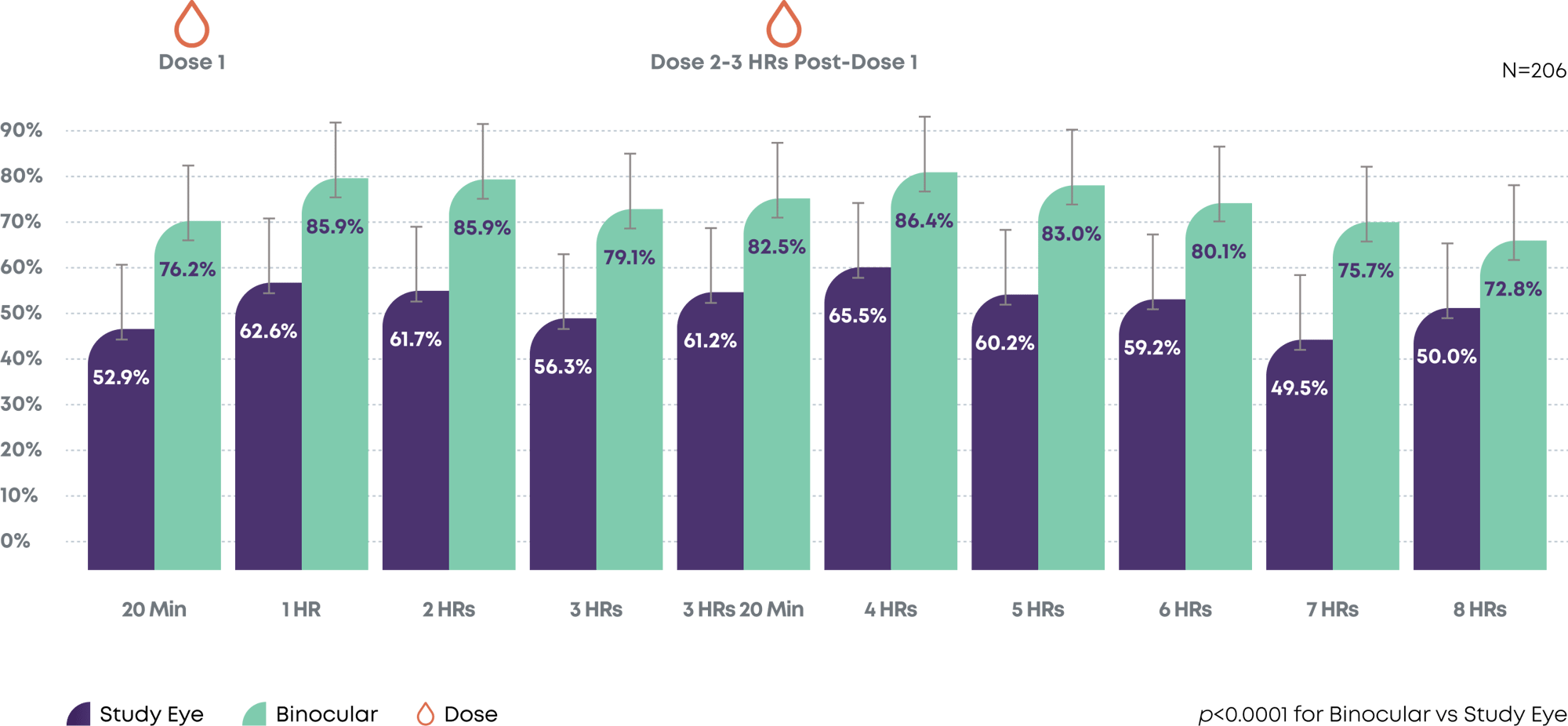
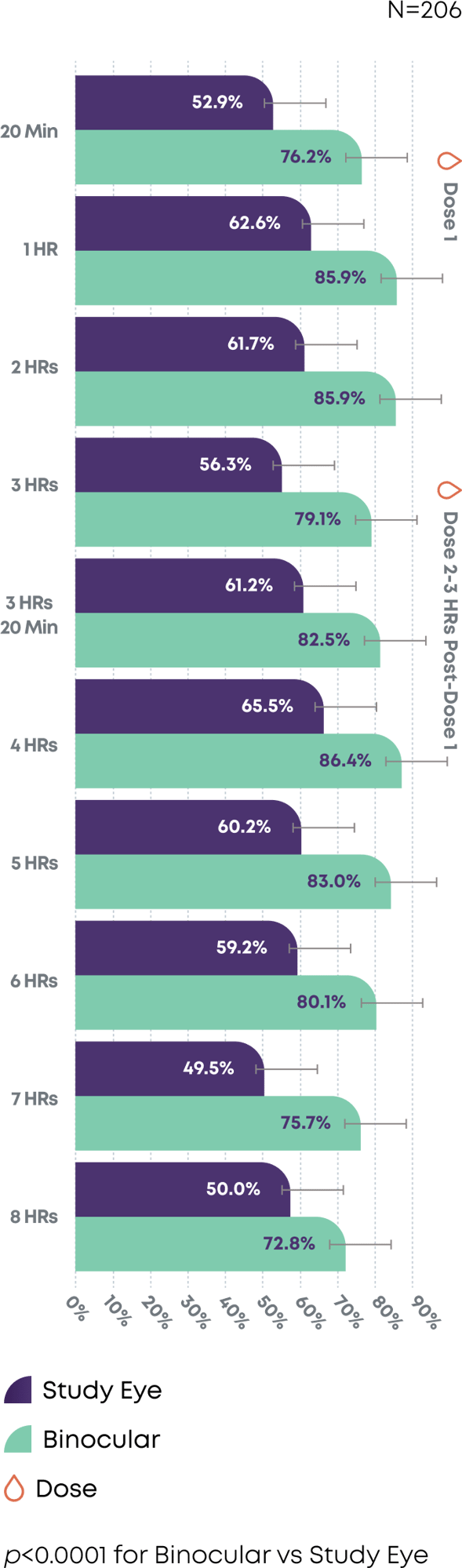
DCNVA=distance-corrected near vision; OU=oculus uterque
See the Study Design below.
Helping patients see where Qlosi fits in
These quick questions can help identify when Qlosi is the right first choice.
When does blurry vision or your readers get in the way?
With Qlosi’s flexible dosing, patients can adjust treatment to fit their individual needs.1
Reference: 1. Qlosi [package insert]. Ponte Vedra, FL. Orasis Pharmaceuticals.
How often do you struggle to read in dim light?
Most patients notice improved clear near vision with Qlosi; Qlosi was tested in mesopic and low luminance lighting conditions.1,2
References: 1. Qlosi [package insert]. Ponte Vedra, FL. Orasis Pharmaceuticals. 2. Holland E, Karpecki P, Fingeret M, et al. Efficacy and safety of CSF-1 (0.4% pilocarpine hydrochloride) in presbyopia: pooled results of the NEAR phase 3 randomized, clinical trials. Clin Ther. 2024;46(2):104-113. doi:10.1016/j.clinthera.2023.12.005
Do you notice yourself needing brighter light or more distance to read comfortably?
By achieving an ideal pupil size, Qlosi helps patients see clearly up close without the need for extra light or readers.1
Reference: 1. Qlosi [package insert]. Ponte Vedra, FL. Orasis Pharmaceuticals.
Which hobbies or activities do you enjoy that require near vision?
Most patients achieve 20/40 functional near vision or better with Qlosi.1,2
References: 1. Cunningham D, Koetting C, Lang J. Comparison of monocular and binocular near vision improvement in the NEAR-1 and NEAR-2 phase 3 randomized, clinical trials: CSF-1 (0.4% pilocarpine HCl) for the treatment for presbyopia. Poster presented at AOA Optometry Meeting; June 21–23, 2024; Washington, DC. 2. Jackson M, Farid M. Pupil size reduction and percentage of participants achieving functional near vision in presbyopia: NEAR Phase 3 clinical trials. Poster presented at: American Academy of Ophthalmology 2025; October 18–20; Orlando, FL.
Does wearing readers affect your confidence?
With Qlosi, patients can see up close without reaching for readers, and with minimal risk of redness.1,2
References: 1. Qlosi [package insert]. Ponte Vedra, FL. Orasis Pharmaceuticals. 2. Holland E, Karpecki P, Fingeret M, et al. Efficacy and safety of CSF-1 (0.4% pilocarpine hydrochloride) in presbyopia: pooled results of the NEAR phase 3 randomized, clinical trials. Clin Ther. 2024;46(2):104-113. doi:10.1016/j.clinthera.2023.12.005
How disruptive would side effects like headache or redness be for you?
Qlosi is a miotic eye drop where in clinical trials, fewer than 10% of patients reported experiencing any single adverse effect.1
Reference: 1. Holland E, Karpecki P, Fingeret M, et al. Efficacy and safety of CSF-1 (0.4% pilocarpine hydrochloride) in presbyopia: pooled results of the NEAR phase 3 randomized, clinical trials. Clin Ther. 2024;46(2):104-113. doi:10.1016/j.clinthera.2023.12.005
Designed to meet the diverse needs of presbyopia patients2
Qlosi’s formulation and flexible dosing make it the ideal first choice for adults seeking clear near vision that fits seamlessly into everyday life
The NEAR-1 and NEAR-2 Qlosi clinical trials2

.png?width=670&height=1979&name=Study%20Design_Mobile%20(1).png)
Examination of the retina is advised in all patients prior to the initiation of therapy.
aFor the first week, the second dose was administered 2 hours post-dose 1. For the second week, the second dose was administered 3 hours post-dose 1.
Primary endpoint:2
- The primary endpoint was the achievement of a ≥3-line improvement (or 15 letter gain) from baseline in DCNVA at 40 cm with no loss in CDVA ≥1-line (or > 5 letter gain) at 4 m on Day 8 at 1 hour post-dose 1
Key secondary endpoints:2
- Key secondary endpoints were the same criteria on Day 8 at 2 hours post-dose 1 and 1 & 2 hours post-dose 2
Key Inclusion Criteria:1
- Age 45-64 years
- Approximately between 20/50 and 20/160 Snellen in at least 1 eye*
- MR between -4.50 and +2.00 D sphere in each eye with <2.00 D difference between eyes
- <2.00 D of cylinder in each eye
- ~20/20-2 Snellen or better CDVA in each eye†
Key Exclusion Criteria:1
- Prior refractive surgery or IOL implantation
- Average dark-adapted pupillometry <3.5 mm in either eye
- >7 letters improvement in post-vehicle treatment in monocular DCNVA in either eye at visit 1 (pretreatment) and visit 2 (pretreatment baseline)
†≤0.04 logMAR CDVA in each eye.
CDVA=corrected distance visual acuity; DCNVA=distance-corrected near visual acuity; IOL=intraocular lens; MR=manifest refraction; NEAR=Near Eye-vision Acuity Restoration